Product Information
| Brand Name | voyage | Certification | ISO9001,ISO13485,CE | Model | |
| Min.Order | 2000 Piece(s) | Size | CM* CM* CM | Payment Terms | L/C,T/T,WesternUnion |
| Weight | Customized service | Place Of Origin | Nantong, Jiangsu |
Product Description
Intended use
For the rapid qualitative determination of Malaria P.falciparum specific histidine rich protein-2(Pf HRP-2)and malaria P.vivax specific lactate dehydrogenase (pLDH)in human blood as an aid in the diagnosis of Malaria infection.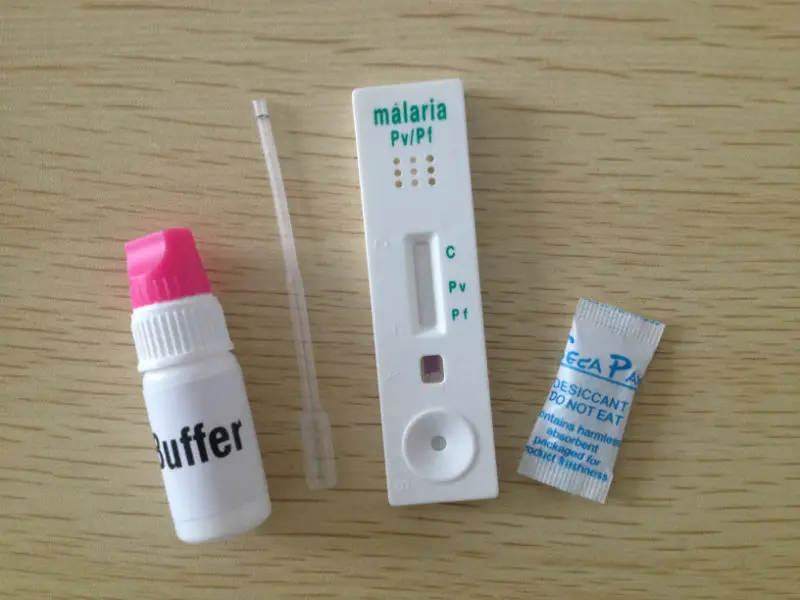
Summary
Malaria is a serious paraitc disease characterized by fever,chills,and anemia and is caused by a parasite that is transmitted from one human to another by the bite of infected Anopheles mosquitoes.There are four kinds of malaria that can infect humans.There four kinds of malaria that can infect humans:Plasmodium falciparum,P.vivax,P.ovale,and P.malariae.In humans,the parasites migrate to the liver where they mature and release another form,the merozoites.
The One-Step Malaria pf(HRPⅡ)/pv(pLDH)Antigen Detection Test Device(Whole Blood) contains a membrane strip,which is pre-coated with two monoclonal antibodies as two separate lines across a test strip.One monoclonal antibody(test line 1)is specific to the p.falciparum histidine rich protein-2(Pf HRP-2) and another monoclonal antibody(test line 2) is pan specific to the lactate dehydrogenase of Plasmodium species (P.falciparum,vivax,malariae,ovale). Conjugate pad is dispensed with monoclonal antibodies conjugated to colloidal gold,which are specific to P.falcparum histidine rich protein-2(Pf HRP-2)and pan specific to the lactate dehydrogenase of Plasmodium species.
Materials
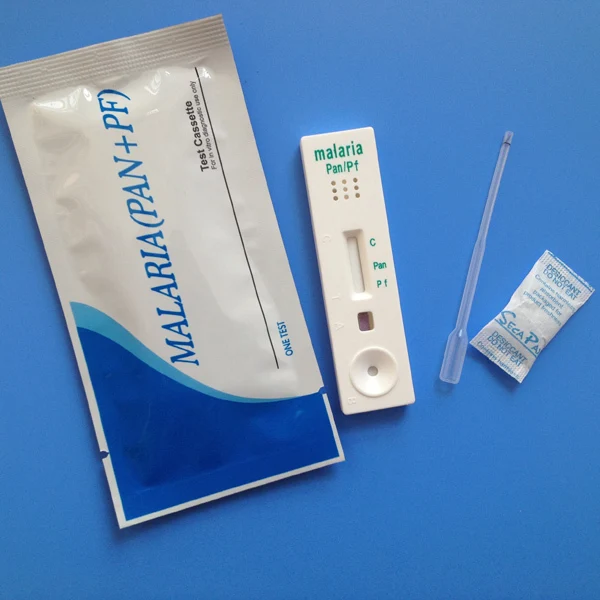
Materials Provided
Test Device
Assay Buffer
Instructions for Use
Materilas lot Provided
Calibrated pipette
Lancet
Timer
Specimen Collection
Collect whole blood into a collection tube (containing EDTA,citrate or heparin) by venipuncture.
If specimens are not immediately tested,they should be refrigerated at 2~8.For storage periods greater than three days,freezing is recommended.They should be brought to room temperature prior to use. Using the specimen after long-term storage of more than three days can cause non-specific reaction.
When stored at 2~8℃,the whole blood sample should be used within three days.
Directions for Use
1. Add 5μl of whole blood into sample well(S),the small well.
2. Add two drops(60μl)of assay buffer into developer well.
3. Read the test result in 20 min.
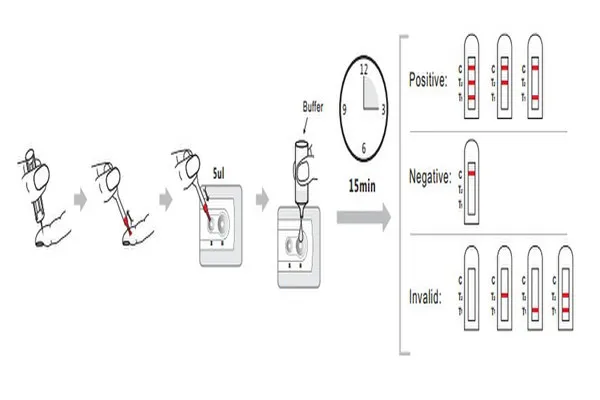
Interpretation of Results
Positive: In addition to a pink colored control (C) band,one or two distinct pink colored bands will also appear in the test (T) region. Please consult your physician to perform a much more detailed exam.
Negative: Only one colored band appears on the control (C) region. No apparent band on the test (T) region.
Invalid: A total absence of color in both regions is an indication of procedure error and/or the test reagent has been deteriorated.
Precautions
For professional in vitro diagnostic use only,Do not use after expiration date.
Do not eat,drink or smoke in the arer where the specimens or kits are handled.
Handle all specimens as if they contain infectious agents.Observe established precoutions against mocrobiological hazards throughout testing and follow the standard procedures for proper disposal of specimens.
Wear protective clothing such as laboratory coats,disposable gloves and eye protection when specimens are being tested.
Humidity and temperature can adversely affect results.
Storage and Stability
The kit can be stored at room temperature or refrigerated(2-30℃)。
The test device is stable through the expiration date printed on the sealed pouch.
The test device must remain in the sealed pouch until use.Do not freeze.Do not use beyond the expiration date.
Campany information
Voyage Medical Co., Ltd is a professional company specialized in diagnostic rapid test devices and hospital beds. With our own factory, we provide worldwide customers the service of made-to-order manufacturing at competitive prices and good quality. Meet customer’s needs and achieve win-win situation has always been the goal of our efforts.

Our company has strong R & D team, rich experience and strong technical strength. The establishment of a joint laboratory with a number of colleges and universities outside the province, give full play to production, science research, combined the advantages of research focused on quick detection products.

At present, voyage has infectious disease detection, tumor marker detection, drug abuse test, pregnancy and eugenics diagnosis and many other series of rapid qualitative diagnosis products, including AIDS, syphilis, hepatitis series test products, home hormone electronic probe, blood glucose test system, immune colloidal gold quantitative detection system, dry quantitative biochemical detection system.
Our Services

Advantage:
1. Popular sell at home and abroad.
2. Nice assessment and feedback.
3. High return rate of single.
After-sales service
Rapid Test Service assurance
Service tenet: quick and decisive and thoughtful thorough and accurate;
Service objectives: service quality to win customer satisfaction;
Service efficiency: the period, we will have a dedicated staff to detect problems with the product for testing, and promptly get back to you;
Service principles: products in the course of product issues caused by improper operation, we will provide a solution.
Packaging & Shipping
To better ensure the safety of your goods, professional, environmentally friendly, convenient and efficient packaging services will be provided.
Related products
FAQ
1.Q:Are you a factory or trading company?
A:We are a trading company with our own factory.
2.Q:Where is your factory located? How can I visit there?
A:Our factory is located in Nantong City, Jiangsu Province, China, near to the Shanghai. You can fly to Shanghai airport directly.All our clients, from home or abroad, are warmly welcome to visit us!
3.Q:What is MOQ?
A: The MOQ depends on different products, generally speaking is 20.000pcs but we can do your requirement.
4.Q:How can I get some samples?
A: We are honored to offer you some free samples.
5.Q:How does your factory do regarding quality control?
A:"Quality is priority. We always attach great importance to quality controlling from the very beginning to the very end. Our factory has gained FSC, ISO13485 and most products have the CE and FDA certificates.
Contact us


Francis XU
automatic electric digital portable hemoglobin test meter price
Certification:ISO9001,ISO13485,CE Min.Order:2000 Other(s)
Rapid diagnostic 7 panel multi Drugs Test drugtest Kits
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
DOA Urine Marjuana THC Drugtest Kit With FDA Marked
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Home-testing rapid diagnostic MOP test kit drug urine testing Strips
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Novel Coronavirus (SARS-COV-2) Antigen Test Kit
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)