Product Information
| Brand Name | Voyage | Certification | ISO9001,ISO13485,CE,FDA | Model | |
| Min.Order | 2000 Other(s) | Size | CM* CM* CM | Payment Terms | L/C,T/T,D/P |
| Weight | Customized service | Place Of Origin | China Jiangsu |
Product Description

Intended Use of Multi Drugs Rapid Test Panel
The DOA Cassettes / Panels are rapid chromatographic immunoassays for the qualitative and simultaneous detection of one to fourteen of the following drugs in a variety of combinations in human urine. The designed cutoff concentrations and direct calibrator for these drugs are as follows:
| Drug Name | Drug Code | Specimen | Cut off Level |
| Marijuana | THC | urine | 50ng / ml |
The DOA Cassettes / Panels are one-step immunoassay in which chemically labeled drugs (drug-protein conjugates) compete for limited antibody binding sites with drugs which may be present in urine. The test device contains membrane strips which are pre-coated with drug-protein conjugates on the test band(s). Each strip, the drug antibody-colloidal gold conjugate pad is placed at one end of the membrane. In the absence of drug in the urine, the solution of the colored antibody-colloidal gold conjugate move along with the sample solution upward chromatographically by capillary action across the membrane to the immobilized drug-protein conjugate zone on the test band region. The colored antibody-gold conjugate then attach to the drug-protein conjugates to form visible lines as the antibody complex with the drug conjugate. Therefore, the formation of the visible precipitant in the test zone occurs when the test urine is negative for the drug. When the drug is present in the urine, the drug/metabolite antigen competes with drug-protein conjugate on the test band region for the limited antibody. When a sufficient concentration of the drug is present, it will fill the limited antibody binding sites. This will prevent attachment of the colored antibody (drug-protein conjugate)-colloidal gold conjugate to the drug-protein conjugate zone on the test band region. Therefore, absence of the color band on the test region indicates a positive result. A control band with a different antigen/antibody reaction is added to the immunochromatographic membrane strip at the control region (C) to indicate that the test has performed properly. This control line should always appear regardless of the presence of drug or metabolite. If the control line does not appear the test device should be discarded.
Principle of Multi Drugs Rapid Test Panel
The DOA Cassettes / Panels are one-step immunoassay in which chemically labeled drugs (drug-protein conjugates) compete for limited antibody binding sites with drugs which may be present in urine. The test device contains membrane strips which are pre-coated with drug-protein conjugates on the test band(s). Each strip, the drug antibody-colloidal gold conjugate pad is placed at one end of the membrane. In the absence of drug in the urine, the solution of the colored antibody-colloidal gold conjugate move along with the sample solution upward chromatographically by capillary action across the membrane to the immobilized drug-protein conjugate zone on the test band region. The colored antibody-gold conjugate then attach to the drug-protein conjugates to form visible lines as the antibody complex with the drug conjugate. Therefore, the formation of the visible precipitant in the test zone occurs when the test urine is negative for the drug. When the drug is present in the urine, the drug/metabolite antigen competes with drug-protein conjugate on the test band region for the limited antibody. When a sufficient concentration of the drug is present, it will fill the limited antibody binding sites. This will prevent attachment of the colored antibody (drug-protein conjugate)-colloidal gold conjugate to the drug-protein conjugate zone on the test band region. Therefore, absence of the color band on the test region indicates a positive result. A control band with a different antigen/antibody reaction is added to the immunochromatographic membrane strip at the control region (C) to indicate that the test has performed properly. This control line should always appear regardless of the presence of drug or metabolite. If the control line does not appear the test device should be discarded.
Pictures
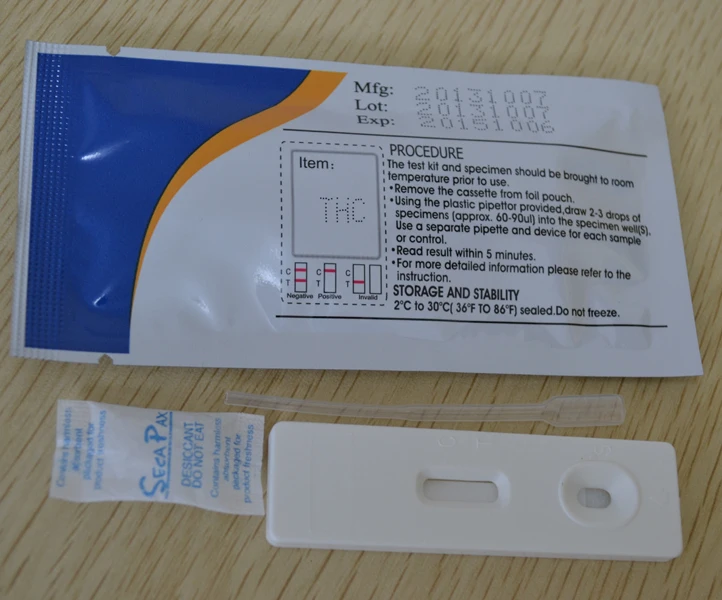
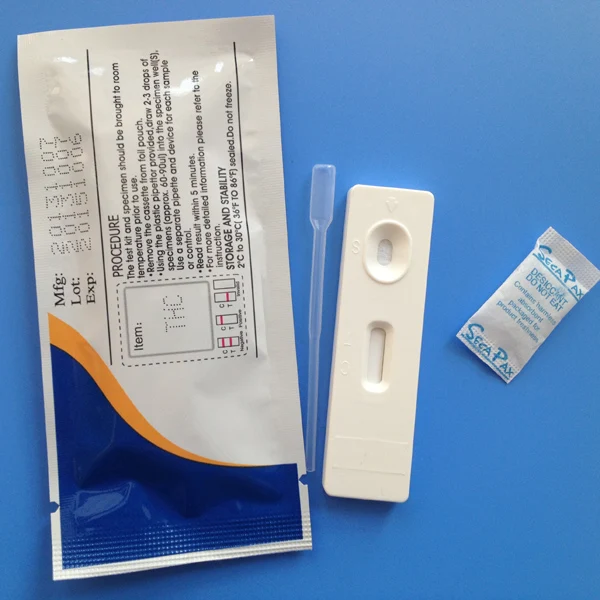
Procedure and Result Reading of THC Test Cassette
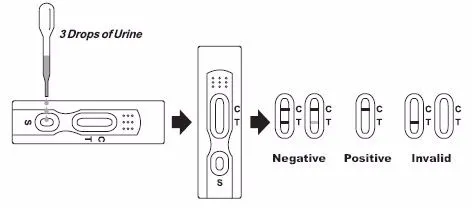
Step 1: Have the donor provide a specimen in the cup. (Collector retains test cup lid.)
Step 2: Verify that temperature is within range on the temperature strip (90° - 100°F).
Step 3: Apply three drops of specimen to each sample well.
Step 4: For test with built-in validity tests, read specimen validity results at the times specified on the color chart provided. If the test pad colors are outside the normal range, collect a second sample, and retest.
Step 5: The test will run in 1-2 minutes. Read the drug test results when the control lines are clearly visible.
Interpreting the Results
| Negative | Negative | Positive | Invalid |




Voyage Medical Co., Ltd is a professional company specialized in diagnostic rapid test devices and hospital beds. With our own factory, we provide worldwide customers the service of made-to-order manufacturing at competitive prices and good quality. Meet customer’s needs and achieve win-win situation has always been the goal of our efforts.




Francis XU
automatic electric digital portable hemoglobin test meter price
Certification:ISO9001,ISO13485,CE Min.Order:2000 Other(s)
Rapid diagnostic 7 panel multi Drugs Test drugtest Kits
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
DOA Urine Marjuana THC Drugtest Kit With FDA Marked
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Home-testing rapid diagnostic MOP test kit drug urine testing Strips
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Novel Coronavirus (SARS-COV-2) Antigen Test Kit
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)