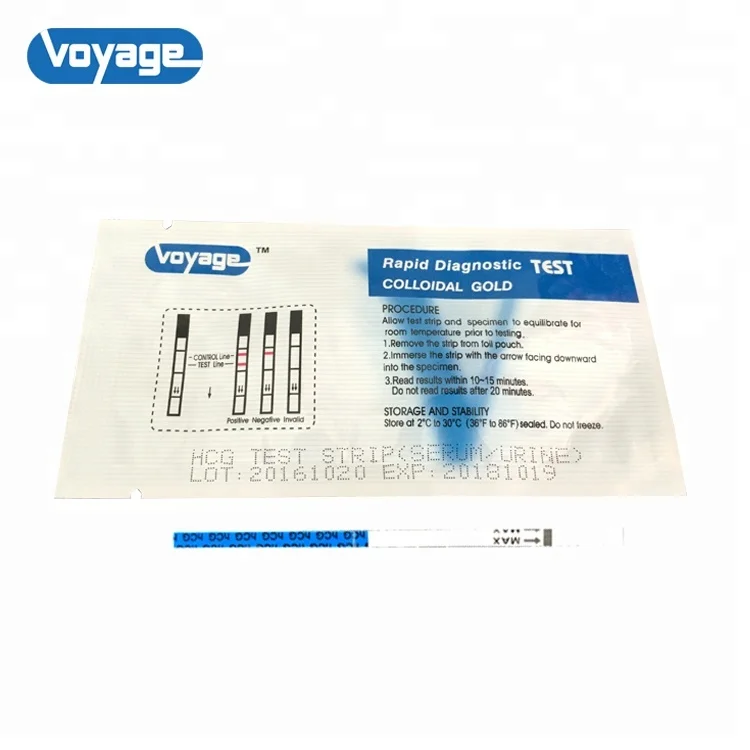
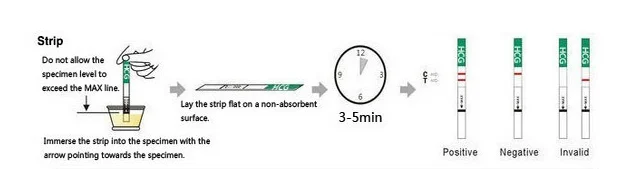
This product is composed of the glass fiber strips of monoclonal antibody against human chorionic gonadotropin (hCG), anti-mouse lgG solid cellulose nitrate membrane and the bonders of absorptive colloidal gold - monoclonal antibody against hCG. It adopts the principles of double antibody sandwichmethod and immunochromatography to test the hCG in the urine.
Intended use Used for early diagnosis of pregnancy



 Ordinary Member Since 2020
Ordinary Member Since 2020  Verified
Verified