Product Information
| Brand Name | Joy Goal | Certification | CFDA,ISO13485,CE | Model | |
| Min.Order | 20000 Other(s) | Size | CM* CM* CM | Payment Terms | L/C,T/T,D/P,PayPal,WesternUnion |
| Weight | Customized service | Place Of Origin |
Product Description
【Product name】
InfluenzaA /InfluenzaB(FluA+B) Antigens Rapid Test Kit (Colloidal Gold)
【Intended use】
The InfluenzaA/InfluenzaB (FluA+B) Antigens Rapid Test Kit is an in vitro diagnostictest for the qualitative detection of influenza A and B nucleoprotein antigensin nasopharyngeal (NP) swab, throat swab samples, using the rapidimmunochromatographic method. The detection is based on the monoclonalantibodies specific for the nucleoprotein of either Influenza virus A or B. Itis intended to aid in the rapid diagnosis of influenza A and B viral infection.Negative results should be confirmed by other methods, such as cell culture.
【Inspectionprinciples】
The Influenza A /Influenza B Rapid Test Kit is animmunochromatographic membrane assay that uses highly sensitive monoclonalantibodies to detect influenza A and B nucleoprotein antigens in nasopharyngeal(NP) swab, throat swab samples. The test strip is composed of the following parts:namelysample pad, reagent pad, reaction membrane, and absorbent pad. The reagent padcontains the colloidal-gold conjugated with the monoclonal antibodies againstinfluenza virus A and B,the reaction membrane contains monoclonal antibodieseither for virus A or for B, and the polyclonal antibodies against the mouseglobulin. The whole strip is fixed inside a plastic device.
When the sample is added into the sample well, conjugates dried in thereagent pad are dissolved and migrate along with the sample. If influenza A orB presents in the sample, a complex formed between the anti-influenza A or Bconjugate and the virus will be captured by the specific anti-influenza A or Bmonoclonal antibodies coated in the T region (T). Results appear at 15 minutes inthe form of a red line that develops in the T on the membrane.
To serve as a procedural control, a red line will always appear in thecontrol region (C) indicating that proper volume of sample has been added andmembrane wicking has occurred
【Main components】
| Specifications | Name of component | Quantity | Main biochemical compositions |
| Card type | Colloidal gold method test paper card of Flu A+Flu B | One card | Include the test papers of influenza A virus antigen and influenza B virus antigen, plastic cards, desiccant, sample extracting tube, dropper and foil bag. The Flu A test paper is consisted of plastic substrates, colloidal gold pad, cellulose membrane, sample pad, and absorption pad. To pre-envelop gold tags of Flu A monoclonal antibodies and rabbit IgG antibody on colloidal gold pad, and respectively pre-envelope Flu A monoclonal antibody and anti-rabbit IgG antibody at the places of test line of nitrocellulose membrane and the control line. The test paper of influenza B virus antigen is consisted of plastic substrates, colloidal gold pad, cellulose membrane, sample adding pad, and absorption pad. To pre-envelop gold tags of Flu B monoclonal antibody and rabbit IgG antibody on colloidal gold pad, and respectively pre-envelope Flu B monoclonal antibody and anti-rabbit IgG antibody at the places of test line of nitrocellulose membrane and the control line. |
| Instructions | One piece | Printing Paper | |
| Sample extraction liquid | One bottle | PH 8.0, 0.01MPBS solution |
【Storageconditions and period of validity】
Avoid light, dry preservation at 2-30℃,nocryopreserving. The period of validity is 24 months since the date ofproduction
【Sample requirements】
Prepare sterile swab used forsampling.
1)NP Swabbing
In the process of samplingnasal secretions, insert the swab into the position of nasal cavity where thereis the most secretions, slightly rotate the swab for seconds , push it inwardto the position where the swab is blocked by the turbinate (about 2.0cm-2.5cmfar from the nostril), and rotate the swab for three times along wall of thenasal cavity, to take the swab out.
2) Throat Swabbing
Sampling of throat secretions: Insert a swabcompletely from the mouth to the throat, with redness parts of the throat walland palate tonsil as the center, hard properly to scrub pharyngeal tonsils onboth sides and pharynx posterior wall, avoid touching the tongue, taking outthe swab.
After sampling, we shall use the virus sample liquidor the sample extracting liquid provided by this kit to treat as soon aspossible. If the sample can not be treated immediately, it shall be stored in adry, sterilized plastic tube, and which shall be sealed strictly, the samplecan be stored for 8 hours at 2℃-8℃, and for a long time at -70℃。
【Test method】
Before testing, the operation instructions (See Figure 1) must becompletely read first.
Before testing, recover all reagents to the room temperature and the testshall be conducted at room temperature.
1、Add sampleextracting solution about 400μl (10 drops) to a sample extracting tubevertically.
2、Insert thesampling swab into the solution of extraction tube, adjoining inner wall of thetube. Rotate the swab for about 10 times, so that the sample is dissolved inthe solution as far as possible.
3、Squeeze swab headof the swab along inner wall of the extraction tube, so that the liquid isstayed in the tube as far as possible, taking out the sample, discard the swaband cover the water dropper.
4、Open the aluminumfoil bag along the tearing port of it, and take out the test card, place ithorizontally.
5、Respectively add 80μl (about 3-4 drops) treated sampleextracts to the both testing holes of the test card.
6、The positive sample can be found within 1-15 minutes,recommend to finally observing and records the test results at the time of 15minutes, and the results showed after 20 minutes have no clinical significance.
|
| |
【Interpretation of the results】(See Figure 2)
Positive: Two purple redstripes appear at the places of detection line and quality control line on thetest strip/test card.
Positive results show that: There is Flu Aand /or Flu B virus antigen in the sample.
Negative: There is onlyone purple red stripe at the quality control line on the test strip/test card.And Negative results show that: NoFlue A and/or Flue B virus antigen can be detected.
Invalid: There is nopurple red stripe at the control line, and which shows that the operationprocess is not right or the kit has been invalid. In this case, please read theinstructions carefully again, using a new test card to detect again. If thereare problems, the use of this batch of products shall be stopped immediately,to contact local supplier.
【Interpretation ofthe results】
1、The purple red stripe at the quality control line is the standard ofinternal control of the kit, and which can be used to determine whether thereis enough samples, whether the chromatography process is normal, whethercorrect operation steps are used, as well as whether the test results areright.
2、When detecting different samples, the purple red stripes at the detectionline can take on different color depths, and which is caused by differentantigen concentrations of Flu A and/Flu B viruses. But, in the specified time,regardless of color depth of the colored tape, even if there is only very weakcolored tape, the sample shall be determined as the positive.
【Test method andlimitations】
1、The kit is onlyused for detecting Flu A and/or Flu B virus antigen in the samples of human nasopharyngealswab and oropharynx swab.
2、Accuracy of thedetection depends on the sampling progress, if the sampling and storage areimproper, or the sample is not fresh, or the sample is conducted freeze andthaw, all above can affect the detection results.
3、If the sample isstored in individual medicines, such as OTC with high concentration andprescription medicines, the detection results may be interfered. If the resultsare suspicious, please test again.
4、The test card canonly be used for qualitative detection of Flue A and/or Flu B virus antigen inthe samples. If you want to detect specific contents of some indicator, pleaseuse relevant special instruments.
5、The positiveresults only show the existence of Flu A and/or Flu B virus antigen in the samples,and which can not be treated the only standard to judge that the organism hasinfected Flu A and/or Flu B virus antigen. And the detection results must bediagnosed by the doctor combining with other clinical symptoms as well as thedetection indicators from other laboratories.
6、If the detectionresults negative, but there are clinical symptoms, we suggest using otherclinical methods for testing. The negative results can not completely eliminatethe possibility of infecting Flu A and /or Flu B virus antigen.
【Performanceindexes of the products】
1)Detection Limit
Minimal detection limit for H1N1 is 755ng/ml.
Minimal detection limit for H3N2 is 1.6ug/ml.
Minimal detection limit for H5 subtype is 1/1000
Minimal detection limit for H7 subtype is 1/1000
Minimal detection limit for H9 subtype is 1/1000
Minimal detection limit for Flu B is 577ng/ml.
2)Cross Reaction
①Influenza A strains
Subtype of H1N1、H3N2、H5、H7、H9 all are positive.
②Influenza B
All are positive forInfluenza B.
③Virus other than influenza
No cross reaction withfollowing pathogens: Adenovirus Type 1~8,11,19,37, Coxsackie virus Type A16,B1~5, Cytomegalovirus, Echovirus Type 3,6,9,11,14,18,30, Enterovirus Type 71,HSV-1, Mumps virus, Parainfluenza virus Type 1~3, Poliovirus Type 1~3, Respiratory syncytial virus, Rhinovirus Type 1A,13,14.
④Mycoplasma etc.
No cross reaction withChlamydia pneumoniae, Chlamydia psittaci, Chlamydia trachomatis, Mycoplasmapneumoniae.
⑤Bacteria
No cross reaction withfollowing bacteria:Acinetobacter baumannii,Bacteroides fragilis, Bordetellapertussis, Candida albicans, Candida glabrata,Cardiobacterium hominis,EikeneUacorrodens, Enterococcus gallinarum, Escherichia.
3)Clinical Study Data Summary
The Influenza A /Influenza B Rapid Test Device Performance vs. Cell Culture for Detection of Flu A.
| Test Sensitivity | |||
| Sample | +/+ | -/+ | %Sens |
|
|
|
|
|
| NP Swab | 18 | 1 | 94.7 |
| Throat Swab | 9 | 4 | 69.2 |
| Overall | 27 | 5 | 84.4 |
| Test Sensitivity | |||
| Sample | +/+ | -/+ | %Sens |
| NP Swab | 83 | 4 | 95.4 |
| Throat Swab | 63 | 4 | 94.0 |
| Overall | 146 | 8 | 94.8 |
The Influenza A /Influenza B Rapid Test Device Performance vs. Cell Culture for Detection of Flu B.
| Test Sensitivity | |||
| Sample | +/+ | -/+ | %Sens |
| NP Swab | 17 | 4 | 81.0 |
| Throat Swab | 10 | 1 | 90.9 |
| Overall | 27 | 5 | 84.4 |
| Test Sensitivity | |||
| Sample | +/+ | -/+ | %Sens |
| NP Swab | 84 | 1 | 98.8 |
| Throat Swab | 66 | 3 | 95.7 |
| Overall | 150 | 4 | 97.4 |
【Descriptions andnotes】
1、The test strip/test card can only be used for in vitro diagnosis test. And which is suitablefor testing the samples of human nasopharyngeal swab, and you may not getaccurate results about other body fluid and samples.
2、In the testenvironment, there shall be not wind, no high temperature and high humidity;the test environment shall be not too dry.
3、When the packageis opened, the test stripe/card shall be tested as soon as possible, to avoidbeing stayed in the air for a long time, resulting in damp and invalid. If theinner package is damaged, the product shall be not used.
4、The kit can bestored at room temperature, avoid damp. The kit stored at low temperature cannot be used until it is balanced to room temperature.
5、Operate accordingto inspection rules of the infectious disease laboratories.
6、If the detectionresults take on negative, while there are clinical symptoms, we shall conductfurther clinical detection. The negative results can not eliminate thepossibility of infecting Flue A/Flu B virus antigen.
7、We can only getinitial screening products using this method; any positive results shall befurther confirmed adopting other methods.
8、 When testing alarge number of samples, pleas make tags, avoid confusion.
9、The test card canonly be used for disposable in vitro diagnosis; and the same test card can notbe used repeatedly. After being frozen or invalid, the test cart must not beused.
10、Avoid using bloody samples

Roger Wong
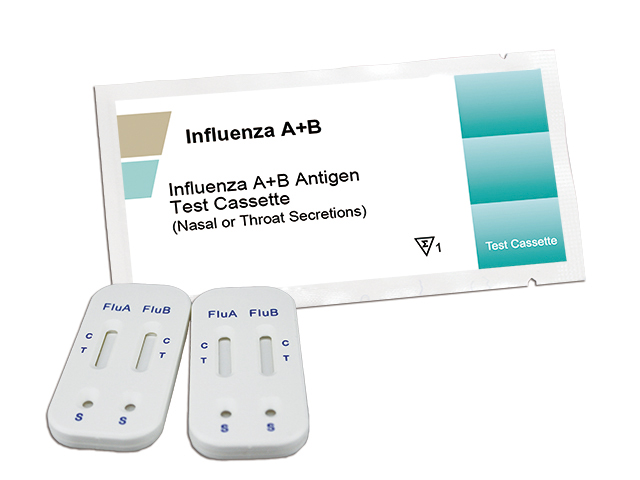
InfluenzaA /InfluenzaB (FluA+B) Antigens Rapid Test Kit (Colloidal Gold)
Certification:CFDA,ISO13485,CE Min.Order:20000 Other(s)