Product Information
| Brand Name | Voyage | Certification | ISO9001,ISO13485,CE | Model | |
| Min.Order | 2000 Other(s) | Size | CM* CM* CM | Payment Terms | L/C,T/T,D/P |
| Weight | Customized service | Place Of Origin | China Jiangsu |
Product Description
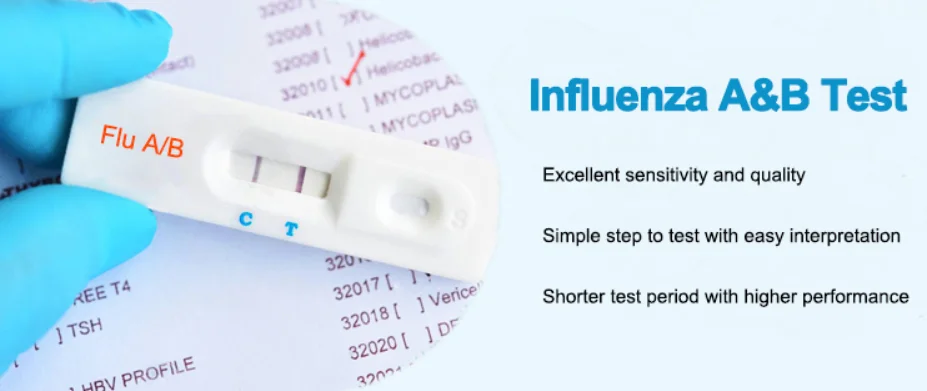
| Product | Influenza virous A B diagnositic rapid test kit cassette |
| Specimen | nasal/throat swab |
| Components | Individually packed test devices,Extraction reagent,Extraction tubes,Steril swabs,package insert |
| Format | cassette |
| Method | colloidal gold |
| Dimension | 3mm,4mm |
| Storage | 4-30℃ |
| Validity | 24 Months |


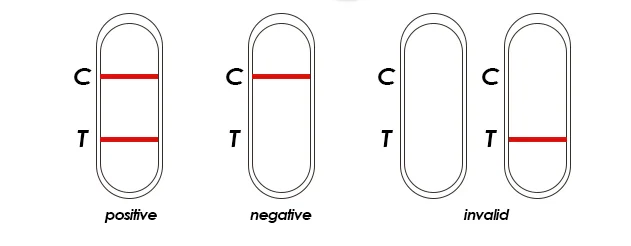







1. Can customers get free samples?
Yes sure. We have plenty types of samples in stock, you can get free sample with freight collect.
2. When can customer get the samples?
3-5 days for stock samples.
3~5 weeks for approved customized samples.
3. Can customer combine many items assorted in one container?
Of course yes.
4. What's MOQ?
Normally it's 10,000pcs. 500pcs for some items.
5. What's lead time?
For stock products, it can be sent to you in 10-25 days .
For OEM products, delivery time is about 3~5 weeks.
6. What's the terms of payment?
T/T, Western Union, Paypal is accepted.
7. What's your shipping way?
Samples by express 3-5 days by door to door,
mass goods by sea to your seaport.
8. How do you control the quanlity?
Doing 100% inspection after production;
then do random inspection before packing;
taking pictures after packing.
9. What about after-sale sevices of Voyage?
We shall response you in 12 hours for your requests.

Francis XU
automatic electric digital portable hemoglobin test meter price
Certification:ISO9001,ISO13485,CE Min.Order:2000 Other(s)
Rapid diagnostic 7 panel multi Drugs Test drugtest Kits
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
DOA Urine Marjuana THC Drugtest Kit With FDA Marked
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Home-testing rapid diagnostic MOP test kit drug urine testing Strips
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Novel Coronavirus (SARS-COV-2) Antigen Test Kit
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)