Product Information
| Brand Name | voyage | Certification | ISO13485,CE,FDA | Model | |
| Min.Order | 2000 Piece(s) | Size | CM* CM* CM | Payment Terms | L/C,T/T,WesternUnion |
| Weight | Customized service | Place Of Origin | Nantong, Jiangsu |
Product Description
For in vitro diagnostic use only.
After suitable amount of urine sample is applied to the Digital Pregnancy Test, the test directly displays the results of“Pregnant" ,“Pregnant>10" “Not Pregnant" or“Error”in the display screen. It is an accurate, rapid, and convenient method for the early diagnosis of pregnancy.
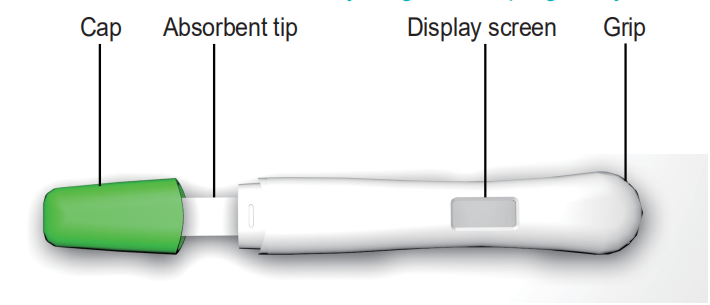
CONTENT OF THE TEST KIT
1. One pouch containing a Digital Pregnancy Test and a desiccant. The desiccant is for storage purposes only, and is not used in the test procedures.
2. Package insert.
WHAT ELSE DO YOU NEED?
A clean, dry, plastic or glass container to collect the urine
PRECAUTIONS
1. This test is for in vitro diagnostic use only. Do not swallow.
2. Discard after first use. The test cannot be reused.
3. Do not use test kit beyond expiration date.
4. Do not use the kit if the pouch is punctured or not well sealed.
5. Keep out of the reach of children.
6. Keep your hand dry and clean before and during testing.
7. Do not use the product out of doors.
8. The procedures should be followed precisely for accurate results.
9. Do not disassemble the battery. The battery is not detachable or changeable.
10. Please follow local regulations to discard used tests.
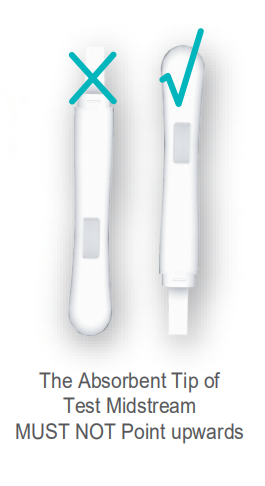
SPECIMEN COLLECTION AND PREPARATION You can test in your urine stream directly, or the urine may be collected with a disposable urine cup or clean container and tested. Any urine specimen is appropriate for the test kit. If the urine specimen cannot be detected immediately, it should be stored at 4°C, and recover to room temperature before use. Urine stored for longer than 2 days is not suitable for the test. Remove a Test Midstream from the foil pouch by tearing at the notch. Hold the grip with one hand and pull out the cap and expose the absorbent with the other hand. Throughout testing never hold the testing Midstream with the absorbent tip pointing upwards. STEP 2: 2. The Hour Glass symbol will appear on the display screen (Refer to Fig.3). HOW TO READ THE RESULTS? LIMITATIONS 6. Please be noted that unclean container or wrong operation will also cause false result.
Note: Do not remove the device from the foil pouch until you are ready to use. To begin testing, read the instruction thoroughly, and do the test under room temperature (15 °C~30 °C).
STEP1:
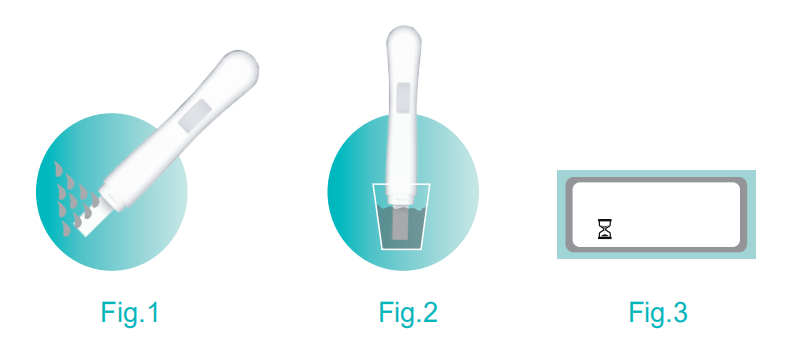
2. The Hour Glass symbol will appear on the display screen (Refer to Fig.3).
3. In about 10 seconds, you will hear the beep sound and the Hour Glass symbol will begin to flash, and then remove the device from the urine stream and keep the Absorbent Tip pointing downwards or lay the Test Device flat. You may wish to replace the cap.
3. In about 10 seconds, you will hear the beep sound and the Hour Glass symbol will begin to flash, and then remove the device from the urine and keep the Absorbent Tip pointing downwards or lay the Test Device flat. You may wish to replace the cap.
Neqative (Not Preqnant):
When the display screen displays“Not Pregnant", it indicates that no pregnancy has been detected.
Positive (Pregnant or Pregnant >10):
When the display screen displays“Pregnant”, it indicates that you are pregnant. If the display screen displays“Pregnant >10", it indicates that you are pregnant for more than 10 days.
Invalid (Error):
When the display screen displays“Error", it indicates that the test fails, is invalid or is used. Repeat with a new test.
1. As it is with any diagnostic procedure, a confirmed pregnancy diagnosis should only be made by a physician after evaluating all clinical and laboratory findings.
2. The device is only suitable for human urine.
3. If a urine sample is too dilute (ie, low specific gravity) it may not contain a representative level of HCG. If pregnancy is still suspected, another urine specimen should be collected 48 hours later and tested.
4. Low concentration of HCG in a very early pregnancy can give a negative result. In this case, another specimen should be obtained at least 48 hours later and tested.
5. Any medical procedure which involves hCG,such as fertility treatment, may cause false positive results. Consult your physician.
1. When should/ test with a Digital Pregnancy Test?
Normally The Digital Pregnancy Test can be used 2 days before your expected period.
2. Which time of urine is best for the dletection?
Any urine specimen is appropriate for pregnancy testing but the first morning urine specimen is optimal because of its highest concentration of HCG.
3. I have used the Test, but the Hour Glass symbol has not appeared. What does this mean?
The Test has not worked correctly. You should repeat the test with a new device or contact distributor or the store, where you bought the product, with the lot number.
4. How to identify whether the power is enough to run a test?
Do the test strictly according to the procedure specified in the instruction. If there is no response in the display screen, it indicates the battery is power off.
5. Could any medication or medical conditions affect the result?
Always read the manufacturer's instructions for any medication you are taking before conducting the test. Fertility drugs containing HCG can give misleading results. These fertility drugs are usually given by injection, and testing too soon after administration may give a false“Pregnant" result. Other fertility therapies,painkillers and hormonal contraceptive (e.g. birth control pills) are unlikely to affect the result. If you are in or approaching the menopause you may obtain a false positive result even though you are not pregnant Ectopic pregnancy and ovarian cysts can give misleading results. If you get unexpected results you should discuss them with
your doctor.
6. What should/ do when my Test says I m Pregnant?
If your result is“Pregnant" or“Pregnant > 10”, you should see your doctor who can advise you on what steps you should take next.

Francis XU
automatic electric digital portable hemoglobin test meter price
Certification:ISO9001,ISO13485,CE Min.Order:2000 Other(s)
Rapid diagnostic 7 panel multi Drugs Test drugtest Kits
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
DOA Urine Marjuana THC Drugtest Kit With FDA Marked
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Home-testing rapid diagnostic MOP test kit drug urine testing Strips
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Novel Coronavirus (SARS-COV-2) Antigen Test Kit
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)