Product Information
| Brand Name | voyage | Certification | ISO13485,CE | Model | |
| Min.Order | 2000 Piece(s) | Size | CM* CM* CM | Payment Terms | L/C,T/T,WesternUnion |
| Weight | Customized service | Place Of Origin | Nantong, Jiangsu |
Product Description
INTENDED USE
The Dengue NS1 Antigen Test is a qualitative test for the detection of dengue virus in human serum, plasma or whole blood. This test is for in-vitro diagnostic use only.
INTRODUCTION
Dengue virus belonging to the Flavavirus group of viruses, is one of the most significant mosquito-born diseases in the world in terms of morbidity and mortality. Transmitted principally by the mosquito types Aedes aegypti and Aedes albopictus, the virus is found commonly throughout the tropic and subtropic regions of the world. There are four known serotypes of dengue. Symptoms of dengue fever include high fever, headache, muscle pain and skin rash. The complications often associated with this infection are dengue hemorrhagic fever or dengue shock syndrome.There are four known distinct serotypes(dengue virus 1,2,3 and 4).NS1 is a highly conserved glycoprotein that is present at high concentrations in the sera of dengue. Infected patients during the early clinical phase of the disease.NS1 antigen is found from the first day up to 9 days after onset of fever in sample of primary or secondary dengue infected patients.
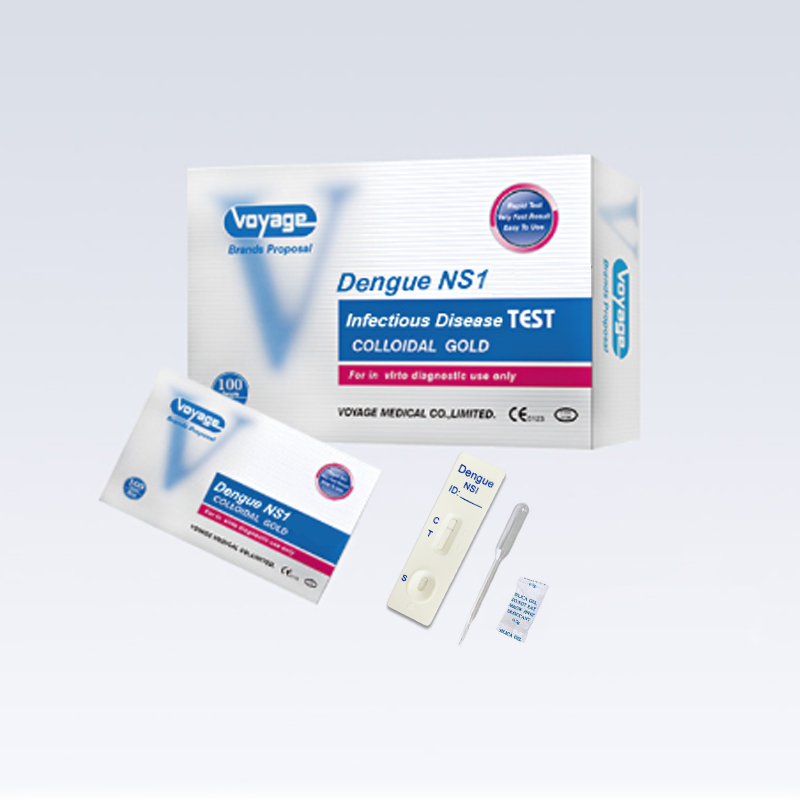
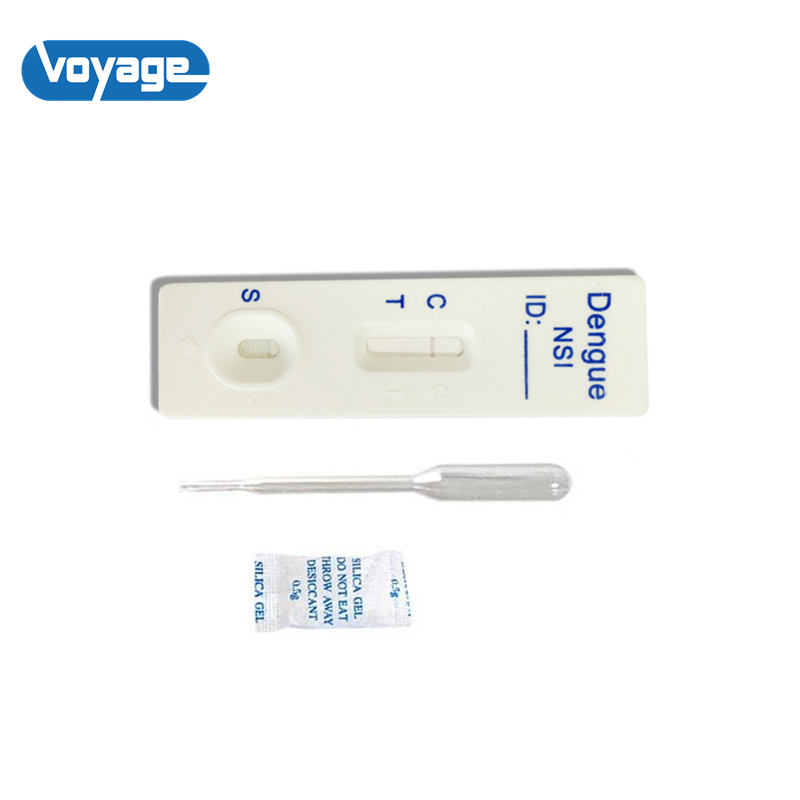
REAGENTS AND MATERIALS PROVIDED
1. Insert Each kit contains 1-50 test devices, each sealed in a foil pouch with three items inside:
1 cassette device.
1 plastic dropper.
1 desiccant.
2. Sample diluent
3. One package insert (instruction for use).
STORAGE AND STABILITY
All reagents are ready to use as supplied. Store unused test device unopened at 2°C -30°C. If stored at 2-8°C, ensure that the test device is brought to room temperature before opening. The test device is stable through the expiration date printed on the sealed pouch. Do not freeze the kit or expose the kit over 30°C.
SPECIMEN COLLECTION
Consider any materials of human origin as infectious and handle them using standard biosafety procedures.
1. Plasma
1. Collect blood specimen into a lavender, blue or green top collection tube (containing EDTA,citrate or heparin, respectively in Vacutainer ) by veinpuncture.
2. Separate the plasma by centrifugation.
3. Carefully withdraw the plasma into new pre-labeled tube.
2.Serum
1. Collect blood specimen into a red top collection tube (containing no anticoagulants in Vacutainer ) by veinpuncture.
2. Allow the blood to clot.
3. Separate the serum by centrifugation.
4. Carefully withdraw the serum into a new pre-labeled tube.
Test specimens as soon as possible after collecting. Store specimens at 2°C to 8°C, if not tested immediately. The specimens could be stored at 2°C to 8°C up to 5 days. The specimens should be frozen at -20°C for longer storage. Avoid multiple freeze-thaw cycles. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible particulate matter should be clarified by centrifugation before testing.
3.Whole Blood
Drops of whole blood can be obtained in a clean container containing anti-coagulant (EDTA,citrate or heparin) by either fingertip puncture or veinpuncture. Do not use any hemolized blood for testing. Whole blood specimens should be stored in refrigeration (2°C -8°C ) if not tested immediately. The specimens must be tested within 24 hours of collection.
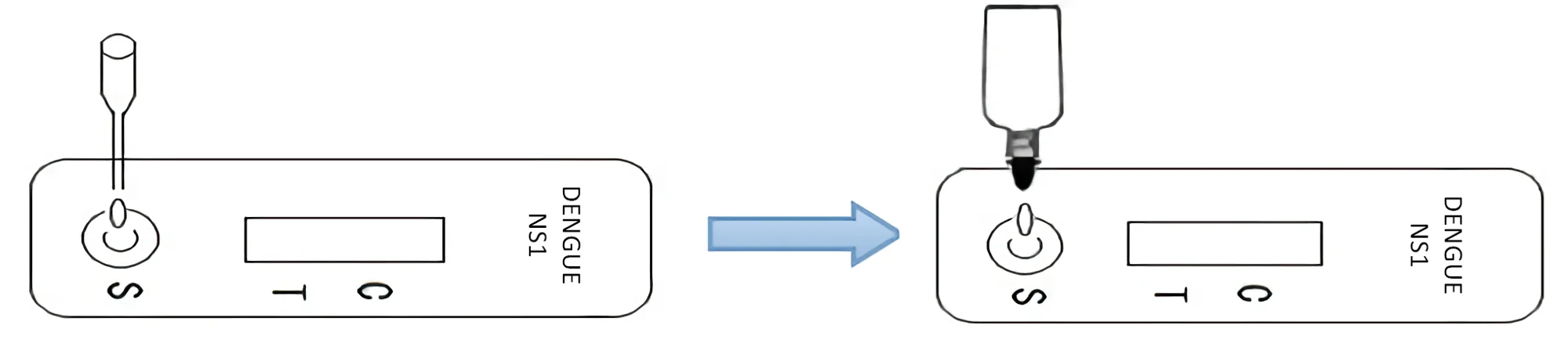
TEST PROCEDURE
Step 1: Bring the specimen and test components to room temperature if refrigerated or frozen. Mix the specimen well prior to assay once thawed.
Step 2: When ready to test, open the pouch at the notch and remove device. Place the test device on a clean, flat surface.
Step 3: Be sure to label the device with specimen’s ID number.
Step 4: Apply 1 drop of specimen (about 30-50 μL) into the sample well.
Then add 1 drop (about 35-50 μL) of Sample Diluent immediately.
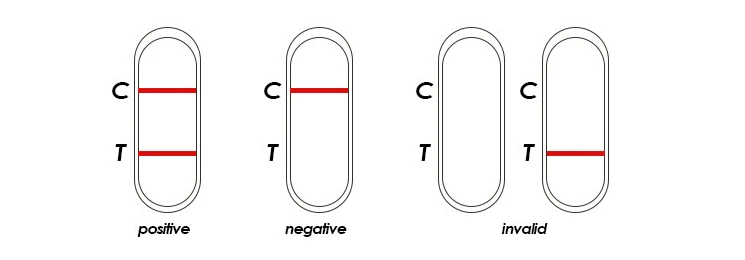
INTERPRETATION OF RESULTS
Negative:
Only control pink band appears on the membrane of the cassette. This indicates that there is no Dengue NS1 Antigen in the specimen.
Positive:
In addition to a pink colored control (C) band, a distinct pink colored band will also appear in the test (T) region.
Invalid:
If without colored band appears at control region, this is an indication of a possible error in performing the test. The test should be repeated using a new one.
PERFORMANCE
1. Performance
Sensitivity:
| Item | Result | |
| Sensitivity | S1 | + |
| S2 | +/- | |
| S3 | - | |
| Negative | 10/10 | |
| Positive | 5/5 | |
2. Cross-reactivity
Test with other Flavivirus mediated and mosquitoes-born disease.
| Disease | Dengue NS1 Negative/Total |
| Japanese Encephalitis | 25/25 |
| Yellow Fever | 25/25 |
| Malaria P.falciparum | 25/25 |
| Malaria P.vivax | 25/25 |
| Total | 100/100 |
The study was conducted to determine the cross-reactivity of the test with positive serum. The following compounds show no cross-reactivity when tested with the Dengue NS1 Antigen Test.
3. Interference-reactivity
| Disease | Dengue NS1 Negative/Total |
| Hemolysis sample(MCHC≤875mg/dl) | 2/2 |
| Yellow jaundice(T-BIL≤169.8μmol/L) | 2/2 |
| Hyperlipidemia(TG≤6.0mmol/L) | 2/2 |
| Rheumatoid factor(≤100U/ml) | 2/2 |
| Total | 8/8 |
The study was conducted to determine the interference-reactivity of the test with above serum, showing no interference-reactivity when tested with the Dengue NS1 Antigen Test.

Francis XU
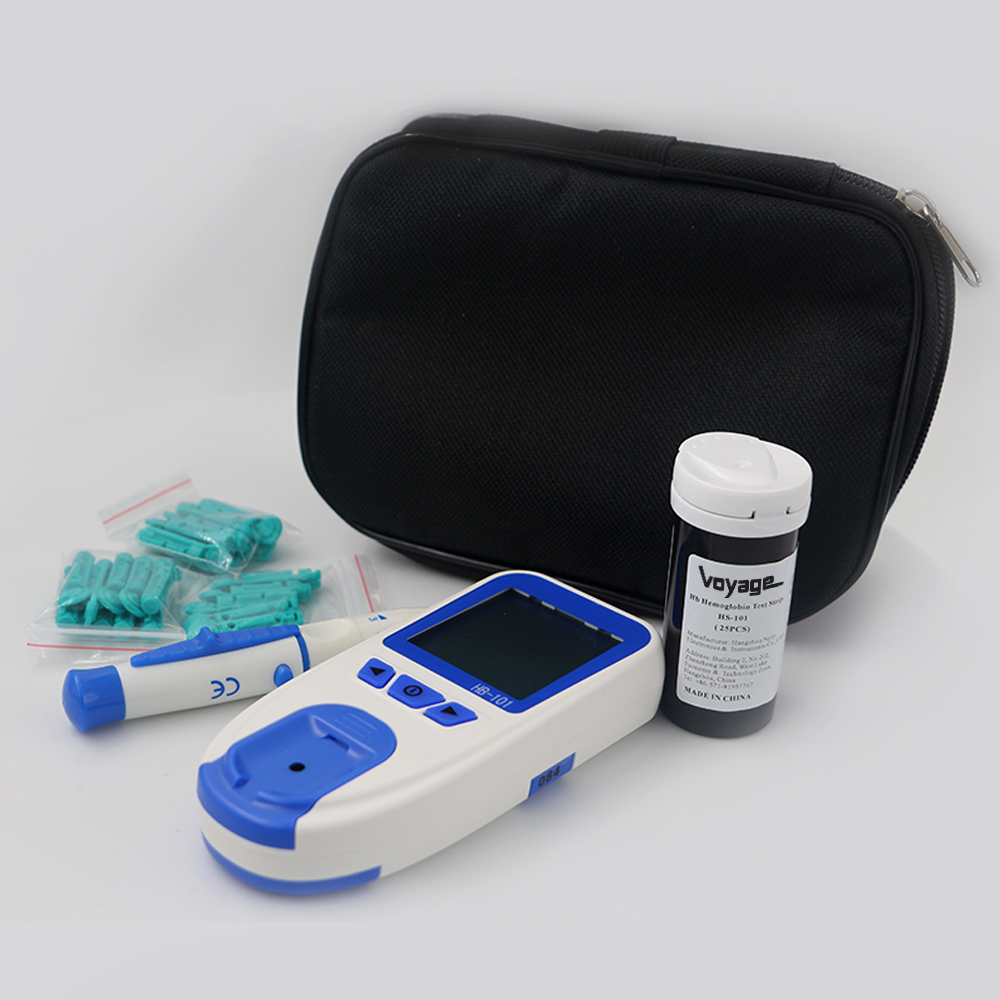
automatic electric digital portable hemoglobin test meter price
Certification:ISO9001,ISO13485,CE Min.Order:2000 Other(s)
Rapid diagnostic 7 panel multi Drugs Test drugtest Kits
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
DOA Urine Marjuana THC Drugtest Kit With FDA Marked
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Home-testing rapid diagnostic MOP test kit drug urine testing Strips
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Novel Coronavirus (SARS-COV-2) Antigen Test Kit
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)