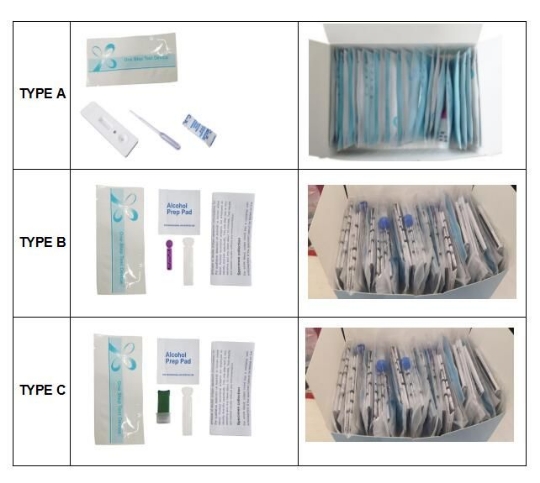
Product Information
| Brand Name | voyage | Certification | ISO13485,CE,FDA | Model | |
| Min.Order | 2000 Other(s) | Size | CM* CM* CM | Payment Terms | L/C,T/T,WesternUnion |
| Weight | Customized service | Place Of Origin | Nantong, Jiangsu |
Product Description
COVID-19 lgG/IgM Rapid Test Kit (Whole Blood/Serum/Plasma) is a solid phase immunochromatographic assay for the rapid, qualitative and diferential detection of lgG and IgM antibodies to 2019 Novel Coronavirusi in human whole blood, serum or plasma. This test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 lgG/lgM Rapid Test kit (Whole Blood/Serum/Plasma) must be confirmed with altemative testing method(s) and clinical findings.
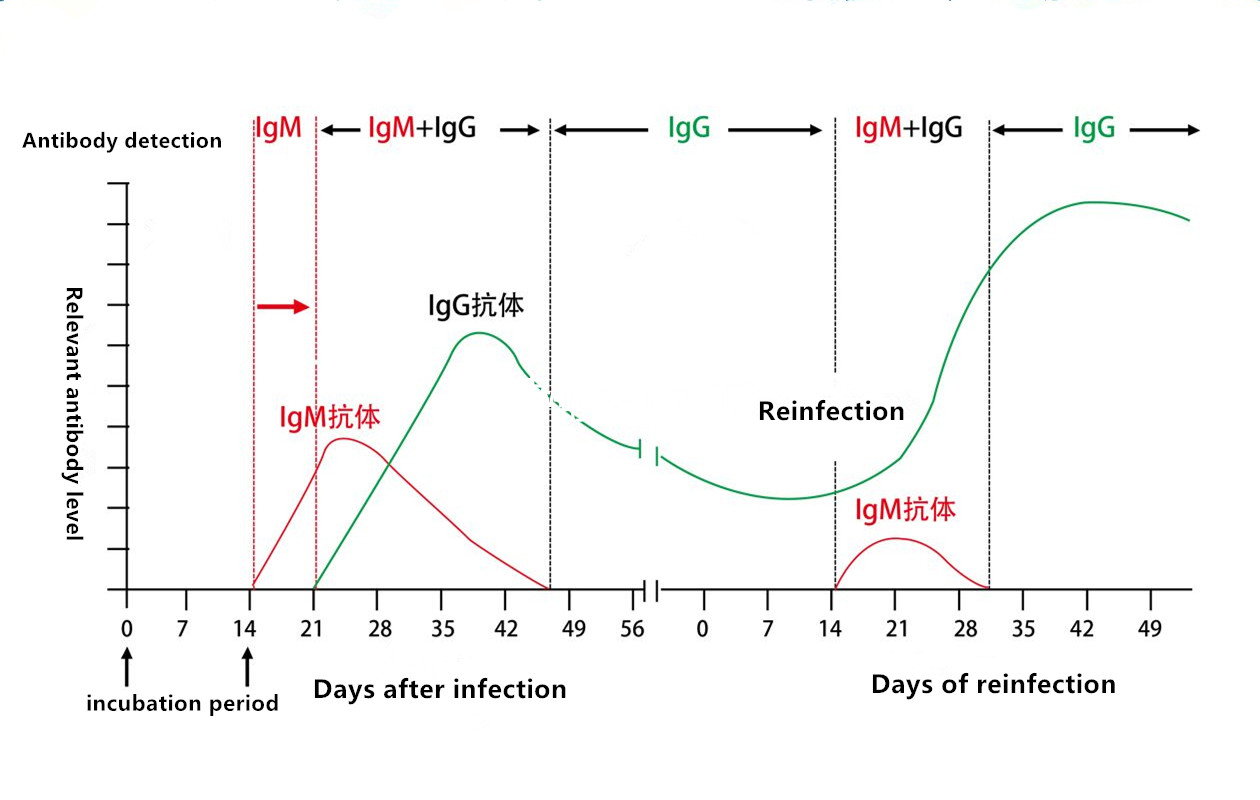
Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals, and birds and that cause respiratory, enteric, hepatic, and neurologic diseases. Seven coronavirus species are known to cause human disease. Four viruses - 229E, OC43, NL63, and HKU1 - are prevalent and typically cause common cold symptoms in immunocompetent individuals.4 The three other strains - severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and 2019 Novel Coronavirus (COVID-19) - are zoonotic in origin and have been linked to sometimes fatal ilness. IgG and IgM antibodies to 2019 Novel Coronavirus can be detected with 2-3 weeks after exposure. IgG remains positive, but the antibody level drops overtime.
PRINCIPLE
The COVID-19 IgG/gM Rapid Test kit (Whole Blood/Serum/Plasma) is a lateral flow immunochromatographic assay. The test uses anti-human lgM antibody (test line lgM) ,anti-human lgG (test line lgG) and goat anti-rabbit IgG (control line C) immobilised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to recombinant COVID-19 antigens conjugated with colloid gold (COVID-19 conjugates) and rabbit IgG-gold conjugates. When a specimen followed by assay buffer is added to the sample well, IgM &/or IgG antibodies if present, will bind tc COVID-19 conjugates making antigen antibodies complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the corresponding immobilized antibody (anti-human lgM &/or anit-human lgG) the complex is trapped forming a burgundy colored band which confim a reactive test result. Absence of a colored band in the test region indicates a non-reactive test result. The test contains an intemal control (C band) which should exhibit a burgundy colored band of the immunocomplex goat anti rabbit lgG/rabbit lgG-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
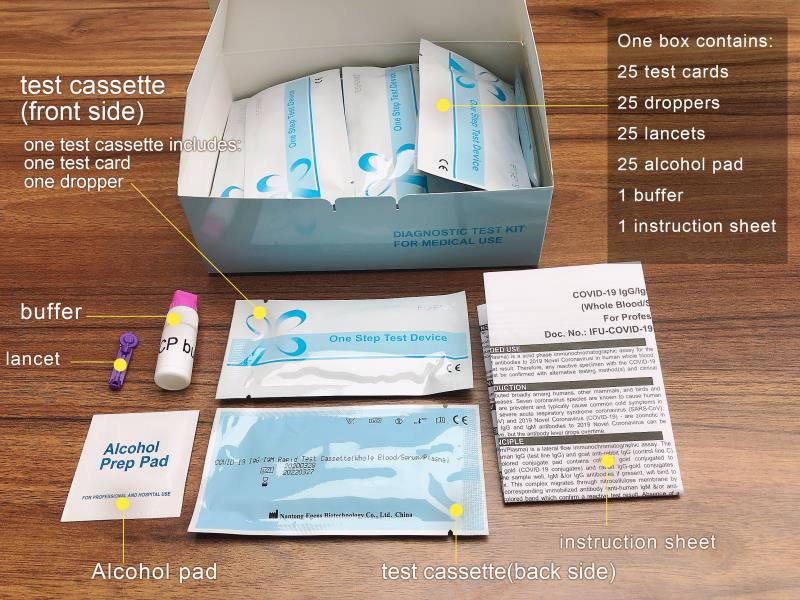
MATERIALS SUPPLIED
Sealed pouches each containing a test kit, a 5. μL mini plastic dropper and a desiccant 1 Buffer
1 package insert
L ancets
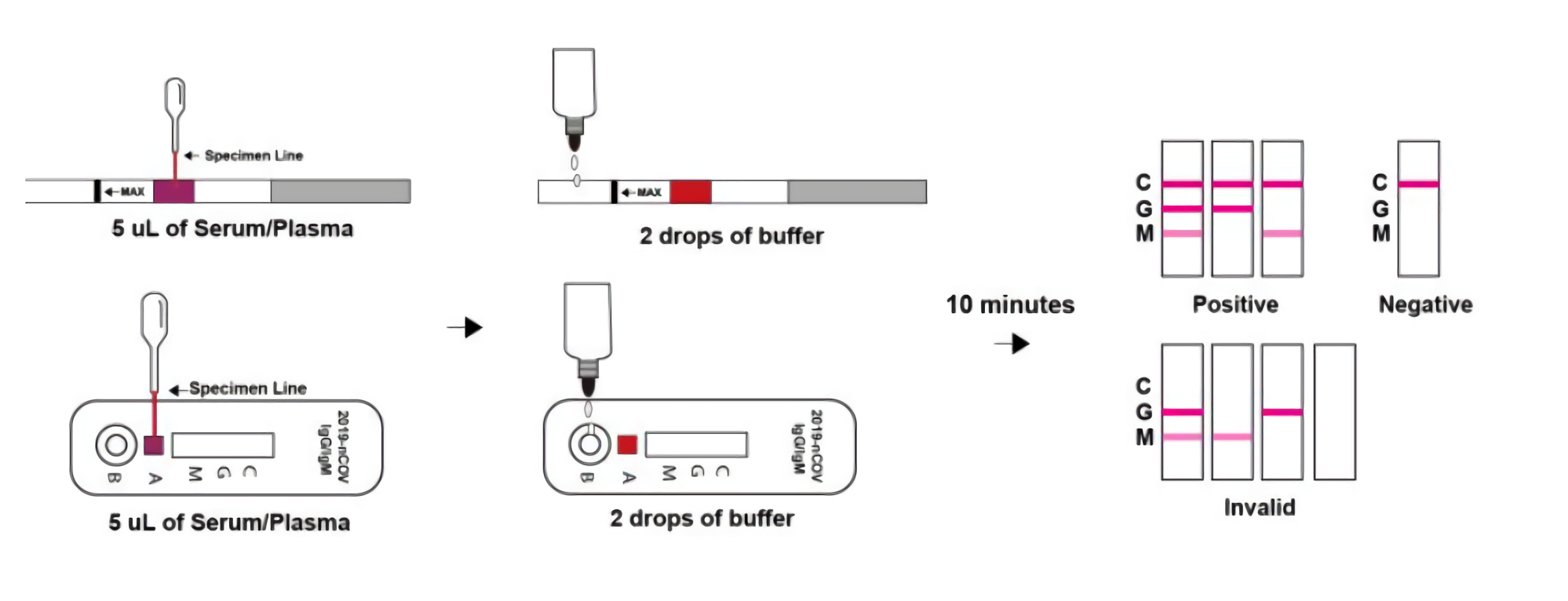
TEST PROCEDURE
For Serum or Plasma Specimens:
Allow test kit, specimen, buffer and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
1. Remove the test strip/cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test kit on a clean and level surface.
Strip:Add 5uL of serum/plasma to the sample pad(purple place with Colloidal gold) of the test strip,then add 2 drops (about 60 μL) of sample buffer to the buffer pad (top of the strip) immediately.
Cassette: Add 5uL of serum/plasma to the specimen well(A) of the test cassette, then add 2 drops (about 60 μL) of sample buffer to the buffer well (B) immediately.
3. Wait for the colored line(s) to appear. The result should be read at 10 minutes. Positive results may be visible as soon as 2 minutes. Do not interpret the result after 15 minutes.
For Whole Blood Specimen
Allow test kit, specimen, buffer and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
1. Remove the test strip/cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface.
Strip:Add. 5uL of whole blood to the sample pad(purple place with Colloidal gold) of the test strip,then add 2 drops (about 60 μL) of sample buffer to the buffer pad (top of the strip) immediately.
Cassette: Add. 5uL of whole blood to the specimen wel(A) of the test cassette, then add 2 drops (about 60 μL) of sample buffer to the buffer well (B) immediately.
3. Wait for the colored line(s) to appear. The result should be read at 10 minutes. Positive results may be visible as soon as 2 minutes. Do not interpret the result after 15 minutes.
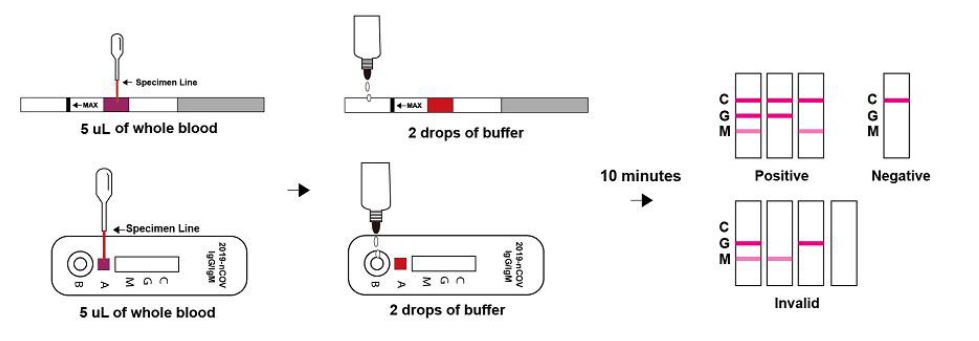
INTERPRETATION OF RESULTS
NEGATIVE: If only the C band is present, the absence of any burgundy color in the both T bands (IgG and lgM) indicates that no anti-COVID-19 antibodies are detected in the specimen. The result is negative.
IgM POSITIVE:
In addition to the presence of C band, if only IgM band is developed, the test indicates for the presence of IgM anti- COVID-19 in the specimen. The result is lgM anti-COVID-19 positive.
IgG POSITIVE:
In addition to the presence of C band, if only lgG band is developed, the test indicates for the presence of lgG anti-COVID-19 in the specimen. The result is lgG anti-COVID-19 positive.
IgG and lgM POSITIVE:
In addition to the presence of C band, both lgG and lgM bands are developed, the test indicates for the presence of both IgG and lgM anti-COVID-19 in the specimen. The result is IgG and IgM anti-COVID-19 positive.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test kit. If the problem persists, discontinue using the test kit immediately and contact your local distributor.

Francis XU
automatic electric digital portable hemoglobin test meter price
Certification:ISO9001,ISO13485,CE Min.Order:2000 Other(s)
Rapid diagnostic 7 panel multi Drugs Test drugtest Kits
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
DOA Urine Marjuana THC Drugtest Kit With FDA Marked
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Home-testing rapid diagnostic MOP test kit drug urine testing Strips
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)
Novel Coronavirus (SARS-COV-2) Antigen Test Kit
Certification:ISO9001,ISO13485,CE,FDA Min.Order:2000 Other(s)